September 24th 2013
By: Sayer Ji, Founder

The U.S. food industry is notorious for poisoning the very consumers who drive their multi-billion dollar enterprise, even spending millions against their right to informed consent (truthful GMO labeling). So, is it any wonder that this deregulated and increasingly deranged juggernaut is experimenting on its own customer base by exposing them to trillions of toxic nanoparticles?
A new study published in Biomedicine and Pharmacotherapy titled, "Effects of titanium dioxide nanoparticles in human gastric epithelial cells in vitro ," reveals for the first time that the nanoparticle form of the common "whitening" agent known as titanium dioxide is capable of inducing "tumor-like" changes in exposed human cells.[1]
Whereas previous cell research has established that titanium dioxide (TiO2) is cytotoxic (cell damaging),[2] this is the first study of its kind to find exposed cells undergo a 'phenotypal' transition from normal to cancerous cell traits, including an increased rate of cell proliferation and a decrease in programmed cell death – hallmark features of precancerous and/or cancerous cells associated with 'immortalization.'
Owing to the fact that the researchers tested human gastric epithelial cells, a type of stomach cell in direct contact with material we eat, and considering the broad range of drug, personal care and food products nanoparticle TiO2 is commonly used within, the toxicological implications of these findings are deeply concerning.
We Are Already Eating Titanium Dioxide
TiO2 is a naturally occurring oxide of titanium, and has a wide range of industrial applications as a "whitening" pigment in plastics, ceramic glazes and paints. It is used in sunscreens as a UV absorbing "sun protection factor," due to its high refractive index. Most of our risk of exposure comes from its use in toothpaste, drugs and excipient-heavy supplements as a pill coating, and food products, including even milk (to 'improve' appearance and texture).
Indeed, given that TiO2 is found in thousands of consumer products, the odds are that you are already being exposed to a significant quantity of them on a daily basis. As reported by Everydayhealth.com , "You ingest around 100 trillion nanoparticles every day, researchers at Binghamton University and Cornell University say."
So, what are some common brands who use it? Nanotitanium is found in products produced by Jello, Nestlé, M&M's, Mother's, Mentos, Albertson's, Hostess and Kool Aid.
Below is a table from the 2012 E Magazine article "Eating Nano " revealing its presence in common U.S. packaged goods.
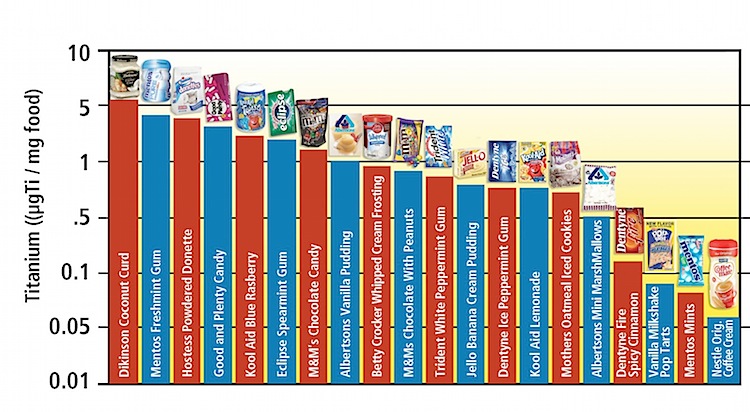
Is Titanium Dioxide Regulated?
Much like present day radiobiological risk assessments for technologies like mammography were developed long before the discovery of DNA, making it impossible to comprehend their DNA-damaging properties at that time, present day biosafety regulations of TiO2 were determined long before the advent of nanotechnology . In both cases, the true harms of these technologies were -- and still are -- greatly underestimated.
As a result of this information gap, TiO2 is currently classified as 'generally recognized as safe' (GRAS) by the FDA, regardless of format. Remarkably, the FDA still allows titanium dioxide in sunscreens "at concentrations of up to 25 percent alone and 2 to 25 percent in combination with any proposed Category I sunscreen active ingredient" without considering the toxicity differential of particle size.[3]Considering that concentrations as low as 0.001% by weight have been found to exhibit clear cytotoxicity within exposed cells,[4] the biosafety regulations governing TiO2 are as great as 5 orders of magnitude or higher less stringent than they should be to protect the consumer.
Nanoparticles are so small they are below the threshold of visibility. This is one reason why they are used for sun protection factor, as 100 nanometers or smaller particles will not leave the white pasty appearance on the skin associated with larger particles. What you can't see, however, is still there – and in the case of nanotitanium, may slip through the surface layers of our skin into more sensitive tissues, as well as our blood stream. This is also why, if you use sunscreen, you should make sure the ingredients say "non-nanoparticle" when describing titanium dioxide or zinc oxide. And this rule applies to purportedly 'natural' brands as well.
Technically, a nanoparticle, also known as a 'ultrafine particle,' is a particle that is sized within the nanometer scale: literally, anywhere between 1-100 billionth (nano) of a meter in diameter. Going up in scale, larger particles are known as 'fine particles,' sized between 2,500 and 100 nanometers, and so-called 'coarse particles' are sized between 10,000 and 2,500 nanometers.
How 'Smaller' Can Indicate A Much Larger Problem
Nanotechnology inverts the unsophisticated logic of conventional toxicology risk assessments: namely, that the smaller the amount of something (concentration or size), the less harmful it is. We have seen how this logic has failed with petrochemical-derived chemicals like benzene , considered toxic in the parts-per-trillion range, and endocrine disrupters like bisphenol A and parabens , which exert powerful hormone-mimicking properties that sometimes increase as their concentration decrease. More recently, Monsanto's Roundup herbicide (glyphosate ), was found to exhibit estrogenicity (and concomitant carcinogenicity) in the parts-per-trillion concentration range . There is also the case of so-called 'low dose' radioisotopes such as depleted uranium , whose relatively low radiolytic decay relative to gamma-ray emitting plutonium generates the illusion that it is safer (recent research performed by the U.S. Army's own Radiobiological Research Institute indicates these "lower risk" radiation sources cause up to a million-fold more damage than present risk models explain due to a phenomenon known as the photoelectic effect).
In other words, less is not only more, but when it comes to particle size, smaller sizes often convey exponentially higher toxicity than larger ones.
Why Are We Not Being Protected?
So, why isn't more being done to protect the consumer from the clear and present health threat represented by nanotechnology? Considering that the Food and Drug Administration does exactly that: administers and/or executes the interests of the food and drug manufacturers, we are supplicating to the wrong entity. The FDA is at least consistent by deciding to allow the food industry to govern itself, but what about the food industry's liability in saturating our food supply with trillions of nanoparticles per serving, without warning the consumer?
According to Tom Philpot, writing for Grist in 2010, "As with GMOs, the strategy seems to be: release into the food supply en masse first; assess risks later (if ever)."
This strategy, while a seemingly successful short-term business model for nanotechnology stakeholders, is utter insanity when one considers the long-term fall out it will have on the industry once millions wake up to the fact they have been treated, once gain, like guinea pigs.
Moreover, as a growing body of peer-reviewed research on the dangers of nanoparticles accumulates , the millions who have already been exposed unknowingly to their ill effects have a legal right to sue for damages. The food industry's increasingly nefarious stance towards the very consumers who ensure their continued business defies logic, and indicates just how unethical their business model really is.
There is really only one answer to this problem. As with unlabeled GMOs, the consumer must refuse to consume them, forcing the manufactures to bow to the holy dollar and reformulate; or, better yet, the 'consumer' must learn how to redefine itself entirely by becoming, once again, a producer, one garden (urban, suburban or rural) at a time. By growing and eating whole foods directly from the earth, we eliminate a wide range of health hazards the mass market food industry has built into their disease-promoting business model.
[1] Monica Catarina Botelho, Carla Costa, Susana Silva, Solange Costa, Alok Dhawan, Paula A Oliveira, João P Teixeira. Effects of titanium dioxide nanoparticles in human gastric epithelial cells in vitro . Biomed Pharmacother. 2013 Aug 23. Epub 2013 Aug 23. PMID: 24051123
[2] GreenMedInfo.com, Research > Problem Substances > Index: T's > Titanium Dioxide
[3] FDA. Sunscreen Drug Products for Over-the-Counter Human Use; Amendment to the Tentative Final Monograph; Enforcement Policy. Federal Register. 1998;63:56584–56589.
[4] Julia X Yu, Thomas H Li. Distinct biological effects of different nanoparticles commonly used in cosmetics and medicine coatings Cell Biosci. 2011; 1: 19. Published online 2011 May 19. doi: 10.1186/2045-3701-1-19






Leave a comment