Aluminum Food Additives Cause Obesity
July 15, 2013
Aluminum is ubiquitous in modern life. Over the last 100 years, the earth has been mercilessly stripped of this useful metal, but little consideration has been paid to the dangers it entails. Now, some scientists are addressing the issue, and their findings can be shocking, as seen in this review documenting how alum devastates the metabolism.

by Heidi Stevenson
A scientific review reveals that aluminum, which is now ubiquitous, damages human metabolism, leading to obesity and chronic liver and neurological disorders—and explains how such metabolic harm happens. The article starts with the statement:
Metal pollutants are a global health risk due to their ability to contribute to a variety of diseases. Aluminum (Al), a ubiquitous environmental contaminant is implicated in anemia, osteomalacia, hepatic disorder, and neurological disorder.
Aluminum is truly ubiquitous in today’s world. It’s about 8% of the earth’s crust, but wasn’t generally a problem until modern times because it was safely sequestered away where it couldn’t produce harm to life. The fact is that aluminum has no place in any biological process in any living organism—but today, virtually everyone is effectively a toxic dumping ground for this poisonous metal.
Because aluminum’s so highly reactive, it’s useful in an enormous range of products and, therefore, mined on a massive scale. It’s common in Agribusiness’ food additives and Big Pharma’s drugs. In no form can aluminum be termed safe. The same characteristic of high reactivity that gives it such a huge range of uses is what makes it so toxic. It’s just as reactive in metabolic processes.
It’s often claimed that aluminum does no harm because kidneys can readily leach it from the body. That’s true—to a point. The kidneys could generally detox aluminum that entered the body naturally, such as through eating plants that grow in acidic soils, like tea. However, their ability to detoxify does have limits, and those limits are now surpassed in most people, with resultant harm to health, including diseases stated in the authors’ introduction: anemia, osteomalacia, and hepatic and neurological disorders, along with the modern-day bane of obesity. This study shows how metabolism is deranged by aluminum, leading to health concerns.
Common Aluminum Sources
Common modern-day food sources of aluminum include:
- Processed cheese, which contains 29.70 mg/gram, as opposed to only 1.57 mg/100g in natural cheese.
- Substitute cream, 13.90 mg/100g
- Baking powder, 2,300 mg/100g (not true of organic baking powder)
Other common sources include:
- Antacids, often taken at the rate of 5,000,000 μg/day
- Industrial air inhalation, 25,000 μg/day
- Antiperspirants, 70,000 μg/day
- Cigarettes, 500-2,000 μg/day
- Vaccines, 1-8 μg/day (though this source is worse because it’s taken almost directly to the brain, as documented by Keele Conference speaker, Romain Gherardi
- Allergy immunotherapy, 7-40μg/day
The authors report that the average daily ingestion of aluminum is about 8 mg/day. Of course, anyone taking most of the antacids on the market, whether prescription or over-the-counter, needs to have second thoughts about it! Antiperspirants are strongly associated with breast cancer, as shown by the research of Dr. Darbre, whose work keeps drawing a stronger and stronger correlation between the aluminum ingredient and cancer.
Aluminum Supplants Iron, Causing Anemia
Aluminum is known to impair iron absorption in the intestines, increase iron serum levels, and disrupt tissue ferritin levels. This review points out that aluminum causes anemia through its ability to supplant iron, whose chemical symbol is Fe. The process is straight-forward:
1. Aluminum displaces iron (Fe) in transferrin, which is a protein that transports Fe in the blood.
2. This results in free Fe in the blood.
3. The body’s Fe-control systems sees the free Fe as an indication that there’s too much iron.
4. Therefore, hepcidin, which is a protein produced by the liver for iron regulation, is secreted.
5. Hepcidin triggers reduction in iron uptake by the intestines and less outflow of iron from cells in the intestine (enterocytes), liver (hepatocytes), and macrophages (type of large white blood cell).
The displacement of iron by aluminum results in excessive amounts of iron in the blood. The body thinks there’s too much, so takes steps to decrease iron levels. The result is blood disorders, and anemia’s lethargy and weakness from inadequate iron.
Reactive Oxygen Species
Aluminum disrupts homeostasis of not only iron, but also metals in general, including magnesium and calcium, which may lead to damage of cell membranes, DNA, and adenotriphosphate (ATP), the form in which energy is produced by cells. The authors state that there’s evidence that even tiny levels of Al can be toxic to neural cells’ genetic material, resulting in increased activation of inflammation and apoptosis.
In spite of all this harm, the authors believe that it’s relatively minor compared to the effects of aluminum on iron homeostasis. The iron supplanted by aluminum leads to the formation of reactive oxygen species (ROS), unstable molecules containing oxygen. The knock-on effects are very serious.
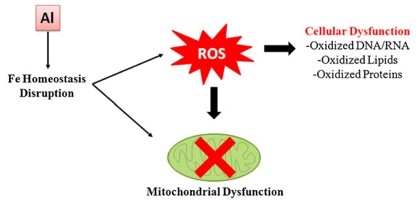
The image to the left is a simple description of the basic nature of damage done by aluminum. By disrupting Fe homeostasis, Al produces ROS, which leads to dysfunction of the mitochondria that exist in all but the red blood cells. Mitochondria are where energy in the form of ATP is produced. This, though is not the only part of cells to be harmed. ROS results in oxidation of DNA and RNA, lipids, and proteins.
Two processes involved in mitochondria energy production are the TCA cycle and the ETC:
- TCA refers to the tricarboxylic acid cycle, which is also known as the Krebs or citric acid cycle. It is a significant part of the ATP production process.
- ECT means electron transport chain. It refers to the process of transporting electrons across the mitochondrial cell membrane in the production of ATP. (Mitochondria have their own cell membranes because they are literally separate living cells, with their own life cycles, inside every cell in your body, with the exception of red blood cells.)
Both TCA and ECT rely on enzymes that contain large numbers of Fe-sulphur bundles, which are seriously depleted by aluminum. Therefore, Al interferes with energy production. The authors point out that both oxidative stress and mitochondrial dysfunction have been linked to the genesis of Parkinson’s disease. Note that excessive ROS, which is caused by Al, are virtually the definition of oxidative stress, so aluminum can cause both of these causative factors in Parkinson’s.
This is continued in How Aluminum Causes Chronic Disease, which includes a stunning list of food additives containing aluminum.
Two of the paper’s authors, Vasu D. Appanna and Joseph Lemire, have presented at the prestigious Keele Conference on the biological effects of aluminum.
Sources:
1. How aluminum, an intracellular ROS generator promotes hepatic and neurological diseases: the metabolic tale (abstract)
2. How aluminum, an intracellular ROS generator promoteshepatic and neurological diseases: the metabolic tale (full text); Cell Biology & Toxicology; Sungwon Han, Joseph Lemire, Varun P. Appanna, Christopher Auger, Zachary Castonguay, & Vasu D. Appanna; DOI 10.1007/s10565-013-9239-0